Bifidobacterium longum
Global patented Bifidobacterium strain with
inhibition of rotavirus infection of infants

-
Isolated from the feces of
healthy breast-feeding infants -
US FDA GRAS Notification
GRN No. 813 -
Deposited at the Korean
Culture Center of Microbiology
as KCCM 10492 -
US Patented
[US20040938162] / Complete
genomic sequenced
Bio-functional
effects
Bifidobacterium longum BORI showed 99% inhibition of the rotavirus infection in the experimental model. It was identified as a single protein named BORI (Bifidobacterium Original Rotavirus Inhibition) by our research team.
Global
Notifications
USA

NDI
(New Dietary Ingredient) approved by FDA [NDI 1082]
USA

GRAS
(Generally Recognized As Safe) approved by FDA [GRN 000813]
USA

Patented strain probiotics.
KOREA

Patented strain probiotics.
KOREA

Halal certificate recognized by Korea Muslim Federation Halal Committee.
Europe

Recognized by the European Food Safety Authority with Qualified Presumption of Safety (QPS)
Australia

Included in “Approved Terminology for Medicines Section – Biological List” by TGA
Canada
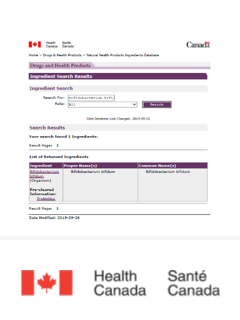
Can be used as Medicinal Ingredients of Natural Health Product
CHINA

Recognized by CFDA used in food
Featured in
book chapters
B. longum BORI, originated from a breast fed infant, is well documented regarding clinical evidence for its health promoting effects by various researchers of universities and hospitals and have been featured in the international textbooks of microbiology.

Encyclopedia of Food and Health

Bergey's Manual of
Systematic Bacteriology:
Volume 5: The Actinobacteria

The Bifidobact
eria and Related Organisms

Advances in Nutraceutical
Applications in Cancer

Carbohydrate-Active
Enzymes
Global
application
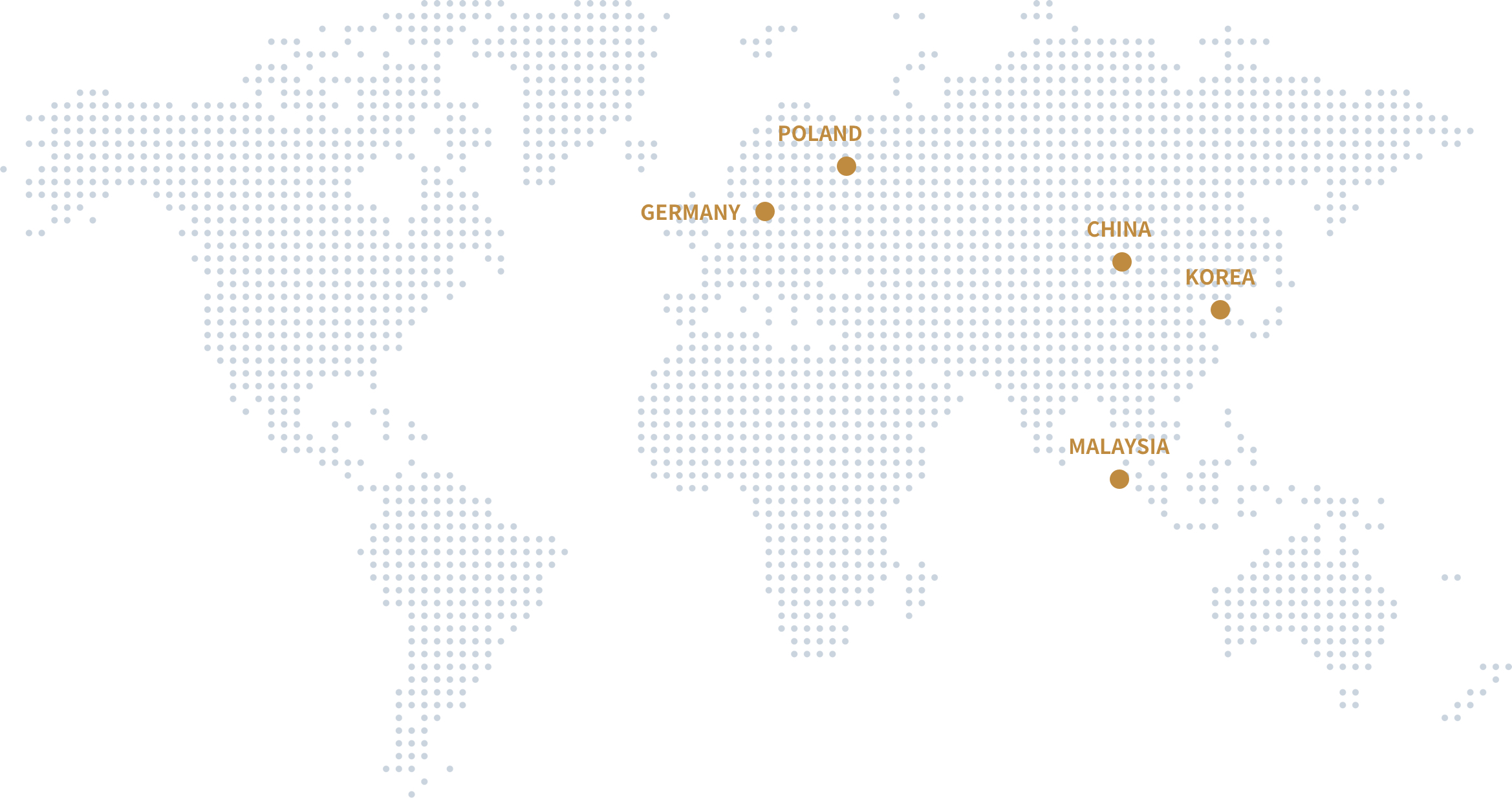
More
References
Certificate
- • Non-GMO
- • BSE-free
- • TSE-free
- • No Allergen
- • Halal Certi
Packaging
- • 1kg / pack
- • 10pack/box
Safety
- • US FDA GRAS approved
- • US FDA NDI approved
- • Whole Genome Sequencing
- • 30 years without side effect
Storage Condition
Be kept in an airtight container
and stored at a temperature not
exceeding 8℃




